Cell-based Trivalent Influenza Vaccine (TIVc) Seqirus (Under 65) Flu
Cell-based Trivalent Influenza Vaccine (TIVc) Seqirus (Under 65) Flu
Product Description
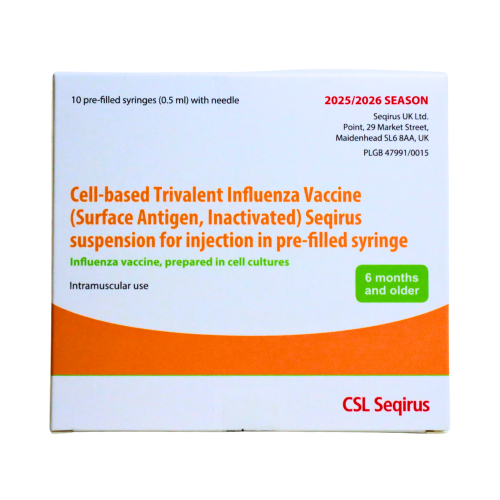
Cell-based Trivalent Influenza Vaccine (Surface Antigen, Inactivated) Seqirus Suspension for Injection in Pre-filled Syringe (single syringe, split pack).
Prophylaxis of influenza in those under 65 years of age.
Reimbursed as part of the NHS Influenza vaccination programme for adults 2025-2026.Directions
Available on request.Ingredients
Available on request.Product Details
Form:Syringe (Pre-filled)Fridge-line:YesMarketing Authorisation:PL-Available on RequestToxin:NoPOM:YesLegal Category:POMFiller:NoStorage Temperature:N/AManufacturer:N/A